Courses
Research Ethics
Atlantic Technological University is committed to ensuring that all research carried out under its name is ethically sound and adheres to the highest standards of research integrity. All research projects must be conducted in accordance with the law, as well as recognised ethical standards. Upholding these standards is essential to protecting the dignity, rights, and welfare of participants, while also safeguarding the credibility of research and the reputation of both researchers and the University. Research that is not ethically robust can place undue burdens on participants or expose them to avoidable risks, which may be physical, psychological, social, or financial.
The ATU Research Ethics Committees play a central role in this process. Their responsibility is to independently review proposed projects to ensure that they are necessary, ethically justified, and designed to minimise potential harm. Formal approval is required for all projects involving human participants or animals.
All staff and students of ATU are required to plan and conduct their research investigations in accordance with appropriate ethical standards. Staff should ensure that they are familiar with any relevant disciplinary guidelines on research ethics and that any empirical research has obtained the required approval from the Research Ethics Committees.
The Research Ethics Committees aspire to provide comprehensive and independent reviews of the ethics of proposed studies, acting in accordance with good ethical practice as dictated by relevant EU Directives, National legislation and practice guidelines. If the proposed research involves patients (i.e. people who are receiving treatment as a result of an illness), the applicant should seek confirmation of the need to submit their application to the relevant hospital’s Research Ethics Committees.
All potential applicants should be familiar with the ATU Research Ethics Policy and the Use of Animals for Research & Teaching Policy.
ATU Research Ethics – Guidance on Applications
Researchers must obtain approval from the relevant Research Ethics Committee (REC) before commencing their project. Apply to the committee that matches your research type:
Submitting to the correct committee ensures that applications are:
- Reviewed by those with the appropriate expertise,
- Assessed at the right level of scrutiny relative to the risks, and
- Processed efficiently without unnecessary resubmission.
University Research Ethics Committee (UREC)
- Research leading to a research degree (e.g., PhD, research Master’s)
- Funded research
- Staff research
- High-risk proposals escalated from FRECs
Applicants are required to review the Research Application Guidance Video before submitting an application to UREC.
Familiarising yourself with its contents is essential, as it outlines key information to support the preparation and submission of your online research ethics application.
Link for Research Ethics Application Form
Contact: urec@atu.ie or brian.doherty@atu.ie ATU Research Ethics Officer
Faculty Research Ethics Committees (FRECs)
- Taught postgraduate research
- Undergraduate research
- Applied learning projects
Contact: Contact your Faculty Office or your Supervisor, who liaises with the FREC.
Animal Research Ethics Committee (AREC)
- All research involving the use of animals (see ATU Policy AQAE025)
Contact: arec@atu.ie
All potential applicants should be familiar with the Research Ethics Policy
Before Applying
Applicants should carefully consider which Research Ethics Committee is the correct body to review their application. ATU operates three committees – the University Research Ethics Committee (UREC), the Faculty Research Ethics Committees (FRECs), and the Animal Research Ethics Committee (AREC) – each with a defined remit.
Submitting to the correct committee ensures that applications are:
- Reviewed by those with the appropriate expertise,
- Assessed at the right level of scrutiny relative to the risks, and
- Processed efficiently without unnecessary resubmission.
This guidance sets out the responsibilities of each committee and clarifies who should apply to which REC.
All potential applicants should be familiar with the Research Ethics Policy
- Does your project involve live animals?
→ ✅ Yes → Apply to AREC (Animal Research Ethics Committee)
→ ❌ No → Go to Step 2
- Are you an ATU staff member OR a postgraduate research student (PhD, MRes, Professional Doctorate)?
→ ✅ Yes → Apply to UREC (University Research Ethics Committee)
→ ❌ No → Go to Step 3
- Are you an undergraduate student OR a taught postgraduate (e.g., MA, MSc) completing a project/dissertation?
→ ✅ Yes → Apply to your Faculty REC (FREC)
→ ❌ No → Go to Step 4
- Is your project funded, externally collaborative, or high-risk (e.g., sensitive data, vulnerable groups, complex interventions)?
→ ✅ Yes → Apply to UREC
→ ❌ No → FREC is usually sufficient
| Please Note: The Research Ethics Application process begins with either a Self-Declaration Form to the FREC or a full application to the UREC. Applications submitted to UREC are screened for completeness, risk-classified, assigned an ID, and reviewed by two reviewers. Link for Research Ethics Application Form UREC Research Ethics Application Pathways Research Ethics applications submitted to ATU’s University Research Ethics Committee (UREC) are assessed according to their level of risk, with two distinct review pathways in place: Low-Risk Applications Low-risk projects follow an expedited review process. Each application is assigned to two independent reviewers, who must both agree that the project meets the required ethical standards. Once approval is confirmed, the Research Ethics Officer (REO) contacts the applicant directly and issues a formal ATU letter of approval. High-Risk Applications High-risk projects are considered in greater depth and are submitted directly to a scheduled UREC meeting for discussion and decision. The outcome of this review process is then formally communicated to the applicant. In all cases, applicants receive official notification of the outcome, and a secure record of the decision is maintained. |
University Research Ethics Committee (UREC)

The University Research Ethics Committee is the central body responsible for safeguarding the highest ethical standards in research at ATU. It provides oversight across the institution, ensures compliance with policy, and reviews research of significant scale or complexity.
Scope:
- Research leading to a research degree (PhD, Master of Research (MRes), Professional Doctorate, etc.).
- Staff-led research projects.
- Funded research.
- Recognition of ethical approvals granted by external institutions.
- Who Should Apply:
- ATU staff.
- Postgraduate research students registered for a research degree (PhD, MRes, Professional Doctorate).
- Researchers applying for funded projects.
- Applicants with high-risk projects (escalated from FRECs).
Link for Research Ethics Application Form
UREC Research Ethics Supporting Documents
UREC Review Outcome
Following review in accordance with the ATU Research Ethics Standard Operating Procedure (Version 1.2), the University Research Ethics Committee (UREC) issues the following decision:
Approved – The application has met all ethical, legal, and institutional requirements. The research may proceed as described.
Contingent Approval – The research may proceed only when the following minor issues have been addressed to the satisfaction of the Research Ethics Office (REO):
Deferred – A decision cannot yet be made. The applicant must provide the following further information or clarification:
Resubmission Required – The application in its current form cannot be approved. A substantially revised application must be submitted, addressing the following issues:
Rejected – The application is considered ethically unacceptable and cannot be approved. The project cannot proceed.
Please email: brian.doherty@atu.ie ATU Research Ethics Officer or urec@atu.ie
Research Ethics Application Flowchart
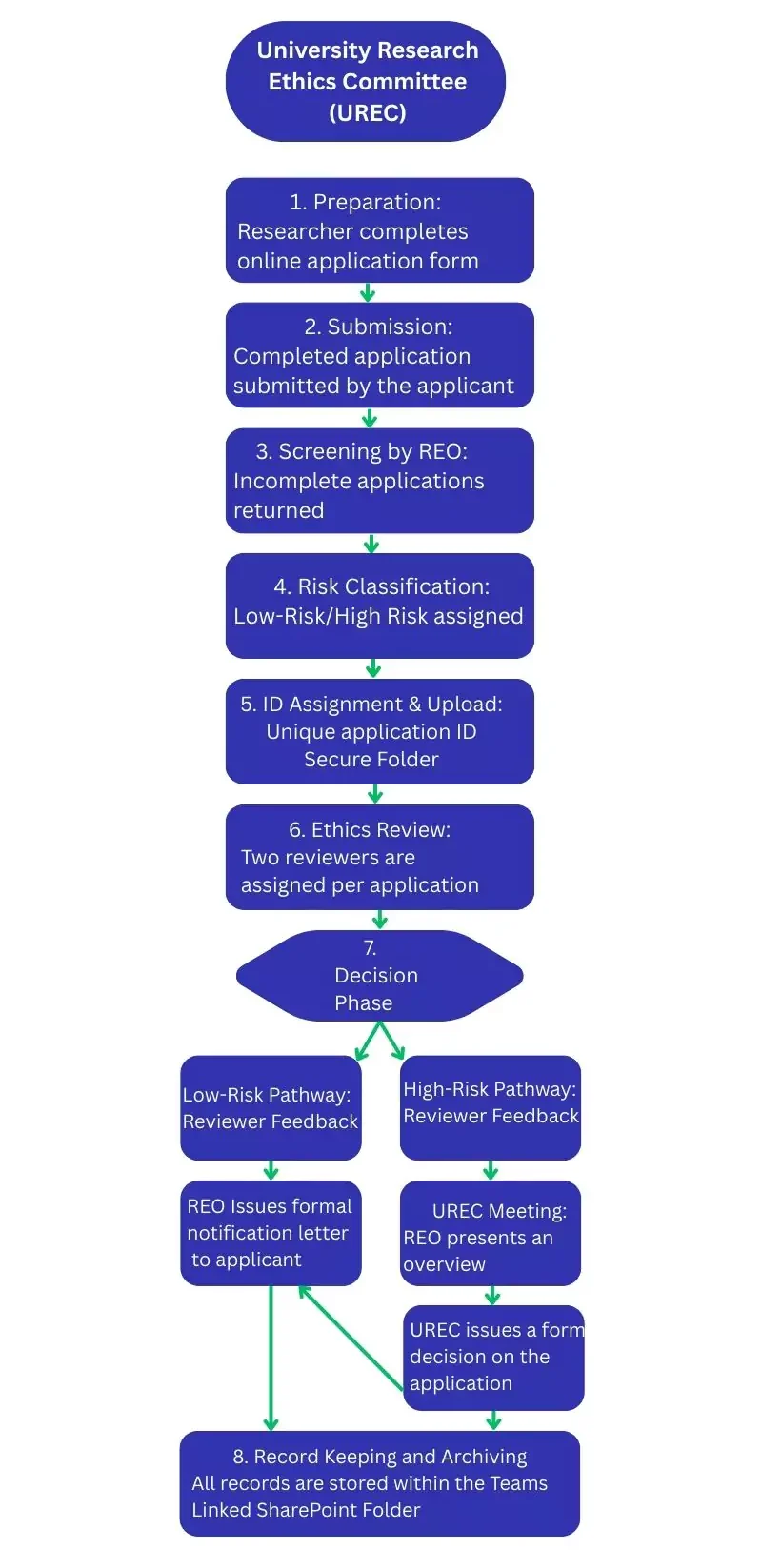
Image Description
Step 1: Preparation of Application
Before submission, the researcher is responsible for preparing a complete application for ethical review. This includes drafting the online Research Ethics Application Form and attaching the relevant supporting documentation.
Supporting Documentation
- Participant Information Sheet (PIS): Provides potential participants with a clear explanation of the study’s purpose, procedures, risks, benefits, and data management practices, enabling them to make an informed decision.
- Informed Consent Form: Records the voluntary agreement of participants, confirming that they understand the nature of the study, associated risks, and their right to withdraw at any time without penalty.
- Supervisor Declaration Form (If Applicable): Confirms that the supervisor has reviewed the proposal, verified its compliance with ethical and legal standards, and accepts responsibility for ongoing oversight.
- Garda Vetting Form (if applicable): Required for research involving children, vulnerable persons, or sensitive environments, ensuring appropriate background checks in compliance with Irish law.
- Export Control Certificate (mandatory): Verifies compliance with Irish and international export control regulations, ensuring that sensitive data, technology, or know-how are not transferred in breach of obligations.
- Data Protection Impact Assessment (DPIA) (if applicable): Required where research involves high-risk data processing, such as special category health data, large-scale personal data collection, or emerging technologies.
- Interview Schedule for Interviews/Focus Groups: A list of key questions or topics used to guide interviews or focus groups.
- Questionnaire/Surveys: Should accompany a research ethics application to show exactly what information will be collected from participants and to ensure the questions are ethical, clear, and appropriate.
- Letter(s) of permission from external organisation(s) granting access to their business, school, charity, database: Letters of permission from external organisations should accompany a research ethics application to confirm that the researcher has formal approval to access the organisation, its data, or participants for the study.
- Any other relevant supporting documents specifically required for your study.
Supervisors must review applications before submission, ensuring completeness, accuracy, and compliance with ATU policy.
Step 2: Submission
The researcher submits the completed application through the designated Microsoft Forms portal, ATU Research Ethics Application form
- Upon submission, the researcher receives an automated confirmation email, including a timestamp, as official evidence that the application has been lodged.
- The supervisor, where applicable, is notified of the submission to confirm their oversight role.
Step 3: Initial Screening
Within two working days of submission, the Research Ethics Office (REO) conducts an initial screening of the application.
- Applications are reviewed for completeness, clarity, and compliance with ATU’s Research Ethics Policy and associated procedures.
- If required information is missing, unclear, or incomplete, the REO returns the application to the researcher with clear written guidance on the amendments needed.
- Applications may only proceed to the next stage once the required corrections have been made and resubmitted.
Step 4: Risk Assessment
Following initial screening, the REO assesses the application to determine whether the project presents a low or high level of ethical risk.
- Risk triggers include involvement of vulnerable populations, use of identifiable or sensitive health data, invasive procedures, or other factors that may increase potential risk to participants.
- Outcome:
- Low-risk applications proceed to expedited review by two academic reviewers.
- High-risk applications are referred to UREC for full committee consideration.
This classification ensures that the level of review is proportionate to the risks involved.
Step 5: ID Assignment and Secure Upload
To ensure traceability and transparency, the REO assigns each application a unique identifier (e.g., UREC-ATU-2025-001).
- All application materials are uploaded by the REO to a secure SharePoint location linked to the University’s Data Management System (DMS).
- This enables tracking of applications from submission through to decision, ensures the integrity of records, and provides an official record for audit and compliance purposes.
Step 6: Ethics Review
All applications, regardless of risk classification, are assigned to two independent academic reviewers.
- Reviewer Assignment: Where possible, applications are allocated to reviewers with expertise in the relevant discipline or methodology.
- Reviewer Anonymity: Reviewer identities remain confidential and are not disclosed to applicants, preserving impartiality.
- Collaboration: Reviewers may work collaboratively when considering an application, but each must submit an independent structured review form.
- Feedback: Reviewer feedback is submitted electronically to the REO, timestamped, and securely stored in the DMS.
This dual-reviewer system ensures fairness, academic rigour, and transparency.
Step 7: Decision Phase
The final decision on an application depends on its risk classification and is informed by reviewer feedback.
· For low-risk applications, reviewers will collaborate to determine whether the application meets the review threshold. If the threshold is met, reviewers can jointly approve the application.
· Decisions by reviewers are communicated to UREC for noting.
Low-Risk Pathway
- Based on reviewer feedback, and under delegated authority of UREC, the REO synthesises comments and issues a formal outcome letter to the researcher.
- Outcome letters confirm the ethical status of the application and are logged in the DMS as the official record.
High-Risk Pathway
The REO formally communicates UREC’s decision to the researcher in writing. This letter serves as the official confirmation and is archived in the DMS.
Applications classified as high-risk, along with reviewer feedback, are scheduled for consideration at the next UREC meeting.
The REO provides UREC with a summary of the application and highlights any key issues raised by reviewers.
UREC deliberates and issues one of five formal decisions:
Approve – application approved as submitted.
Contingent Approval– approval subject to specific modifications or clarifications.
Defer Approval – The committee cannot approve yet because more information or clarification is needed before a decision can be made.
Request Resubmit – The application has major issues and must be substantially revised and submitted again for full review.
Reject – application not approved on ethical grounds.
Step 8: Record Keeping and Archiving
Records Management
All applications, decisions, and correspondence are securely stored in a Teams-linked SharePoint folder.
Please contact brian.doherty@atu.ie for further guidance on the application process
Faculty Research Ethics Committees (FRECs)
Faculty Research Ethics Committees operate within each Faculty to provide accessible and discipline-specific review of student research proposals. They ensure that undergraduate and taught postgraduate projects are carried out ethically, while guiding students and supervisors on best practice.
Scope:
- Undergraduate research projects.
- Taught postgraduate research projects.
- Applied learning projects embedded in modules or industry engagement.
Who Should Apply:
- Undergraduate students undertaking a research project.
- Taught postgraduate students (e.g., MA, MSc) completing a dissertation or project.
Undergraduate/taught postgraduates → Contact your Faculty Office or your Supervisor, who liaises with the FREC.
Research Ethics Application Flowchart
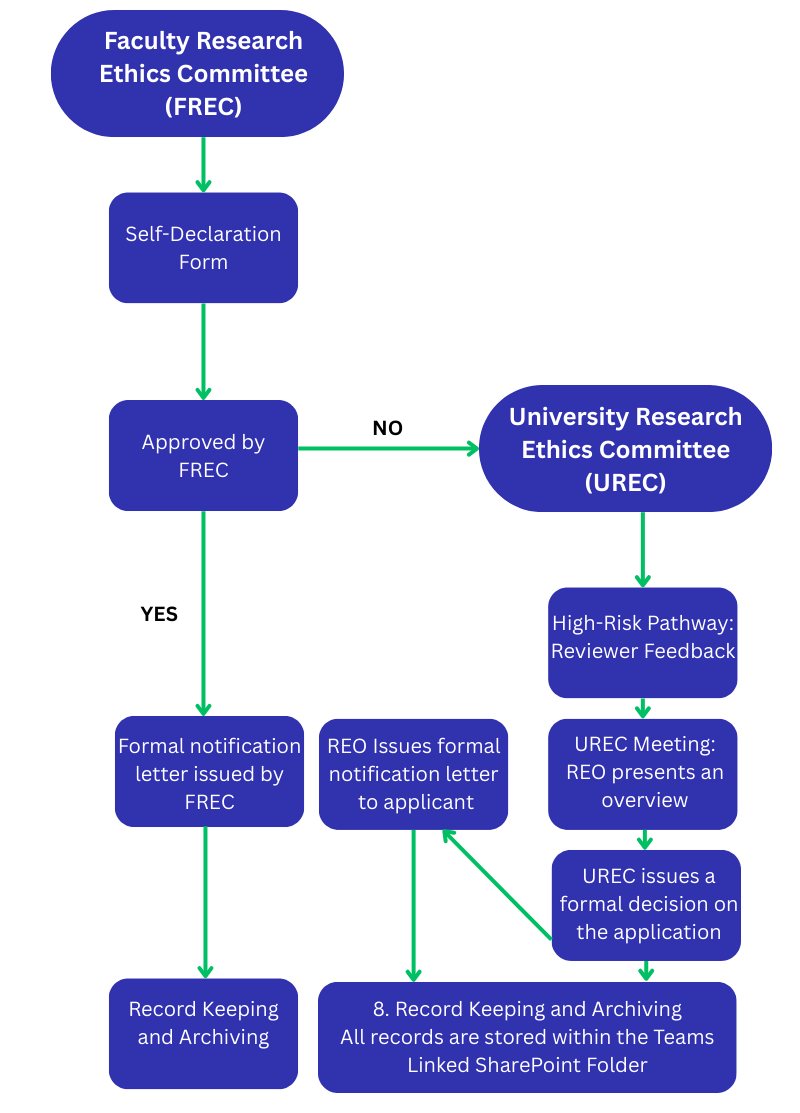
Image Description
The Faculty Research Ethics Committee (FREC) oversees the research ethics applications from undergraduate research and taught postgraduate research programs.
The process is as follows:
1. Application Submission
- The researcher first contacts their Faculty Office or supervisor.
- A Self-Declaration Form is completed and submitted.
2. Faculty-Level Review
- FREC reviews the application.
- If approved, the applicant receives a formal letter of approval.
- If the project is considered high risk, it is escalated to the University Research Ethics Committee (UREC).
When Applications Are Escalated to UREC
Applications are typically referred to UREC if they involve:
High-Risk Participants
- Children or young people under 18
- Adults lacking the capacity to consent
- Prisoners, asylum seekers, or other vulnerable groups
High-Risk Methods
- Clinical interventions, drug trials, or invasive procedures
- Deception without adequate debriefing
- Research that could cause significant psychological distress, harm, or long-term impact
Legal or Ethical Issues
- Work involving the HSE, social services, or healthcare records
- Handling sensitive personal data (e.g., criminal records, immigration status)
- International fieldwork in politically unstable or unsafe regions
Potential Reputational Risk
- Research on highly controversial topics
- Projects likely to attract media or legal scrutiny
UREC High-Risk Pathway
Once escalated to UREC
- Two reviewers are assigned to assess the application.
- The application follows the High-Risk Pathway.
- Reviewer feedback and the application are presented at the next UREC meeting.
- All high-risk applications are considered directly by the committee.
- The Research Ethics Officer issues a formal notification letter to the applicant.
Records Management
All applications, decisions, and correspondence are securely stored.
Please contact brian.doherty@atu.ie for further guidance on the application process
Animal Research Ethics Committee (AREC)
The principles of Replacement, Reduction, and Refinement (the 3Rs) inform all animal-related research and teaching at ATU. The Animal Research Ethics Committee conducts the ethical review of research and teaching involving animals. It ensures that all animal use complies with European and national legislation and that animal welfare is respected through the application of the 3Rs principles (Replacement, Reduction, Refinement).
The Health Products Regulatory Authority (HPRA) is the national competent authority for regulating the use of live animals for scientific or educational purposes. More details on the HPRA are available here
Scope
- Any research or teaching involving animals, or the euthanasia of animals.
- Ethical review before applications for HPRA Individual and Project Authorisations.
Who Should Apply:
- Staff and students using animals in research or teaching.
Please email: arec@atu.ie for information on how to apply for ethical review
Animal Welfare Body
ATU’s Animal Welfare Body is a statutory committee responsible for ensuring the welfare of animals in the establishment and promoting the 3Rs (reduction, replacement and refinement). The AWB plays a crucial role in:
- Advising scientists and staff on animal welfare and the conduct of projects.
- Monitoring projects to identify opportunities for further application of the 3Rs.
- Implementing best practices.
- Advising on re-homing, when feasible.
If you wish to report animal welfare concerns relating to research being undertaken in ATU, please email arec@atu.ie.
AREC Process Flowchart
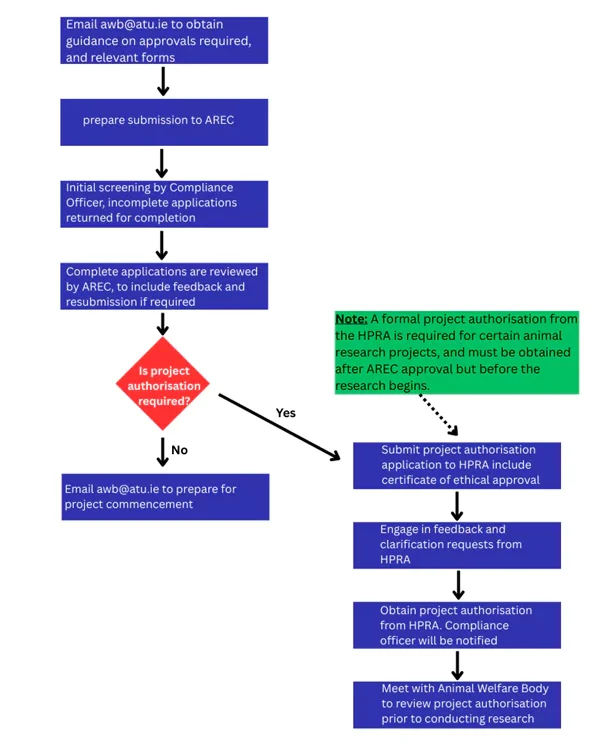
Image Description
The image is a flowchart outlining the process for obtaining project authorization for certain animal research projects. It consists of a series of steps and decision points, presented in a logical sequence.
- Start: The first step instructs the researcher to email awb@atu.ie to get guidance on the necessary approvals and relevant forms.
- Prepare Submission: The researcher then prepares a submission to AREC (Animal Research Ethics Committee).
- Initial Screening: A Compliance Officer conducts an initial screening. If the application is incomplete, it is returned for completion.
- Review by AREC: Once complete, the application is reviewed by AREC. Feedback may be provided, and resubmission might be required.
At this point, the flowchart splits into two paths based on whether project authorization is required:
- If not required: The researcher should email awb@atu.ie again to prepare for project commencement.
- If required:
- Submit a project authorization application to HPRA (Health Products Regulatory Authority), including a certificate of ethical approval.
- Respond to feedback and clarification requests from HPRA.
- Once approved, project authorisation is granted by HPRA, and the Compliance Officer is notified.
- The researcher must then meet with the Animal Welfare Body to review the authorization before starting the research.
Please contact arec@atu.ie for further guidance on the application process
| Meeting Number | UREC Meeting Date | Day | Time | Portal Opens | Portal Deadline (5pm) |
|---|---|---|---|---|---|
| 1 | 01-Oct-25 | Wednesday | 11:00 AM – 12:15 PM | 01-Sep-25 | 17-Sep-25 |
| 2 | 04-Nov-25 | Tuesday | 11:00 AM – 12:15 PM | 18-Sep-25 | 21-Oct-25 |
| 3 | 04-Dec-25 | Thursday | 10:00 AM – 11:15 AM | 22-Oct-25 | 20-Nov-25 |
| 4 | 28-Jan-26 | Wednesday | 11:00 AM – 12:15 PM | 21-Nov-25 | 14-Jan-26 |
| 5 | 25-Feb-26 | Wednesday | 11:00 AM – 12:15 PM | 15-Jan-26 | 11-Feb-26 |
| 6 | 27-Mar-26 | Friday | 10:00 AM – 11:15 AM | 12-Feb-26 | 13-Mar-26 |
| 7 | 30-Apr-26 | Thursday | 11:00 AM – 12:15 PM | 14-Mar-26 | 16-Apr-26 |
| 8 | 28-May-26 | Thursday | 11:00 AM – 12:15 PM | 17-Apr-26 | 14-May-26 |
| 9 | 17-Jun-26 | Wednesday | 11:00 AM – 12:15 PM | 15-May-26 | 03-Jun-26 |
For further queries, contact the following:
UREC Chairperson
Vice President, Regional Development & Engagement
chris.omalley@atu.ie
University Research Ethics Committee
urec@atu.ie
Brian Doherty
Research Ethics Officer
brian.doherty@atu.ie
Animal Research Ethics Committee
arec@atu.ie
Helpful Links and Documents
National and international guidance documents
- International Ethical Guidelines for epidemiological studies WHO
- Operational Procedures for research ethics committees
- HRB policy of research ethics
- Ethics for researchers EUROPE
- inf206 guidelines for internet mediated research
Animal / Genetic research
- HRB Paper on the Legal and Ethical Considerations for Genetic Research
- HRB Policy on use of animals in research
Data Protection
- Webinar-on-GDPR-and-health-research-regulations-2018
- Data protection guidelines on research in the health sector
- Data Protection Act 2003
Vulnerable groups

